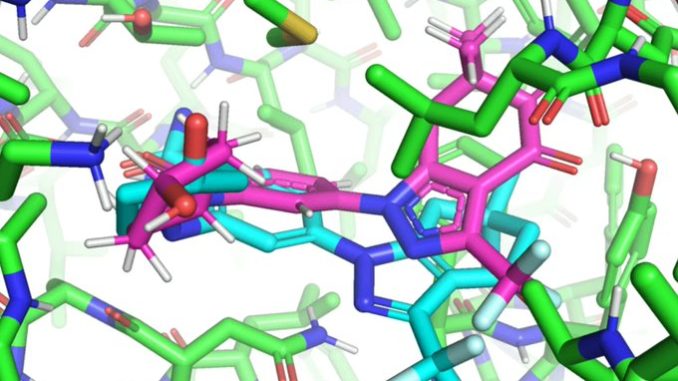
A geometric deep-learning model is faster and more accurate than state-of-the-art computational models, reducing the chances and costs of drug trial failures
The entirety of the known universe is teeming with an infinite number of molecules. But what fraction of these molecules have potential drug-like traits that can be used to develop life-saving drug treatments? Millions? Billions? Trillions? The answer: novemdecillion, or 1060. This gargantuan number prolongs the drug development process for fast-spreading diseases like Covid-19 because it is far beyond what existing drug design models can compute. To put it into perspective, the Milky Way has about 100 thousand million, or 108, stars.
In a paper that will be presented at the International Conference on Machine Learning (ICML), MIT researchers developed a geometric deep-learning model called EquiBind that is 1,200 times faster than one of the fastest existing computational molecular docking models, QuickVina2-W, in successfully binding drug-like molecules to proteins. EquiBind is based on its predecessor, EquiDock, which specializes in binding two proteins using a technique developed by the late Octavian-Eugen Ganea, a recent MIT Computer Science and Artificial Intelligence Laboratory and Abdul Latif Jameel Clinic for Machine Learning in Health (Jameel Clinic) postdoc, who also co-authored the EquiBind paper.
Before drug development can even take place, drug researchers must find promising drug-like molecules that can bind or “dock” properly onto certain protein targets in a process known as drug discovery. After successfully docking to the protein, the binding drug, also known as the ligand, can stop a protein from functioning. If this happens to an essential protein of a bacterium, it can kill the bacterium, conferring protection to the human body.
